
Are you aware of potential rectal side effects from prostate radiation?
If you’re undergoing radiation therapy for prostate cancer, you may be concerned about potential side effects to the rectum like diarrhea, rectal bleeding, and hemorrhoids.
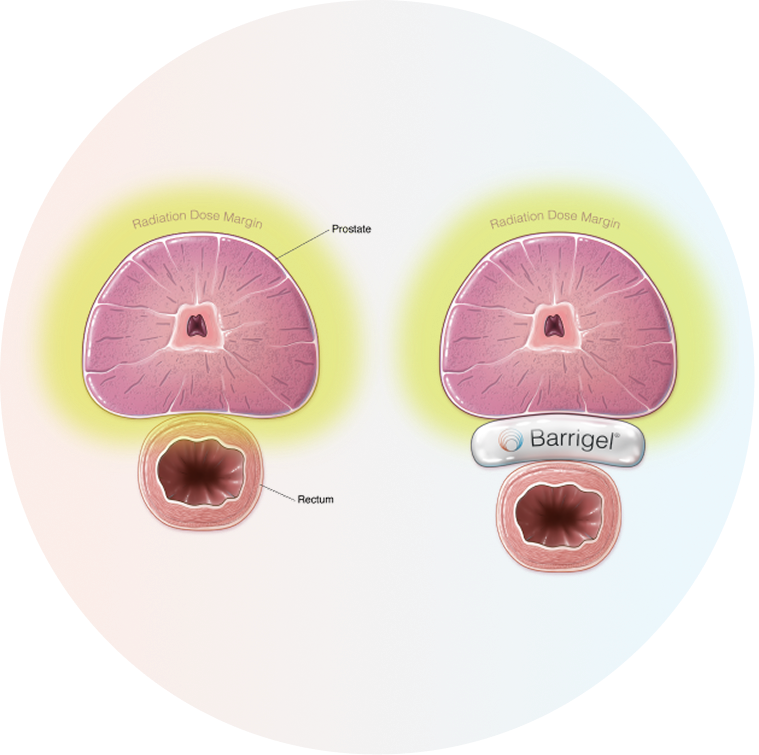
Barrigel™ rectal spacer is the only rectal spacer that can be tailored to your unique anatomy,1,2 protecting the rectum by moving it out of the high-dose radiation area.1
The rectum is at risk for RADIATION EXPOSURE.
More than half of all people with prostate cancer receive radiation therapy3 – however, radiation beams can potentially affect healthy tissue near the prostate and result in long-term radiation side effects. Due to its close proximity to the prostate, the rectum is at risk for radiation beam exposure.
Barrigel™ rectal spacer helps protect the rectum by reducing the amount of radiation that reaches it during treatment.1

Barrigel™Rectal Spacer is CLINICALLY PROVEN to reduce rectal side effects of prostate cancer radiation.1
An even, symmetrical implant reduces unwanted radiation exposure to the rectum.4 Barrigel™ rectal spacer gives the physician control over the shape and placement of the implant.1,2,4,5
The result: Barrigel™ rectal spacer is clinically proven to significantly reduce unwanted rectal radiation exposure in 98.5% of patients.1
“It was great. I had no problems. I feel that it gave me an added layer of protection because I have had no bowel problems. Talk with your doctor and make sure it is right for you. I highly recommend it.”†
Crispin
Real Barrigel™ Rectal Spacer Patient
SAFETY and STABILITY.1
Barrigel™ rectal spacer is made from Non-Animal Stabilized Hyaluronic Acid (NASHA).7
Hyaluronic acid is naturally present in the human body and is highly compatible and fully absorbable. NASHA has a proven history of safety and efficacy in a wide variety of medical applications.*8,9
The procedure is similar to your prostate biopsy and may be performed under local, regional, or general anesthesia—it can be performed in hospitals, outpatient clinics, or doctors' offices prior to the start of radiation treatment.

Barrigel™ rectal spacer is intended to temporarily position the anterior rectal wall away from the prostate during radiotherapy for prostate cancer and, in creating this space, it is the intent of Barrigel™ rectal spacer to reduce the radiation dose delivered to the anterior rectum. Barrigel™ rectal spacer is composed of biodegradable material and maintains space for the entire course of prostate radiotherapy treatment and is intended to be absorbed by the patient’s body over time. Barrigel™ rectal spacer should only be administered by qualified and properly trained physicians with experience in ultrasound guidance and injection techniques in the urogenital/pelvic area. As with any medical treatment, there are some risks involved with the use of Barrigel™ rectal spacer. Potential complications associated with the use of Barrigel™ rectal spacer include, but are not limited to: pain associated with Barrigel™ rectal spacer injection; needle penetration of the bladder, prostate, rectal wall, rectum, or urethra; injection of Barrigel™ rectal spacer into the bladder, prostate, rectal wall, rectum, urethra, or intravascularly; local inflammatory reactions; infection; urinary retention; rectal mucosal damage, ulcers, necrosis; bleeding; constipation; and rectal urgency. More information on indications, contraindications, warnings and instructions for use can be found in the Instructions For Use at www.barrigel.com. Individual results may vary.
Caution: Federal (USA) law restricts this device to sale by or on the order of a physician.
1. Mariados NF, Orio PF III, King MT et al. JAMA Oncol (2023).**
2. King MT, Svatos M, Orio PF III et al. Pract Radiat Oncol (2023).**
3. Mayo Clinical, Radiation therapy, www.mayoclinical.org. Accessed: October 12, 2024. https://www.mayoclinic.org/tests-procedures/radiation-therapy/about/pac-20385162
4. Williams J, Mc Millan K, Bolton D et al. J Med Imag and Radiat Sci (2022).
5. Gejerman G, Goldstein MM, Chao M et al. Pract Radiat Oncol (2023).***
6. Data on File. As of 10/01/2024.
7. Barrigel Injectable Gel Instructions for Use (2022).
8. Restylane® celebrates 25 years of natural-looking results with its signature line of hyaluronic acid fillers. 2021.
9. Svatos M, Chell E, Low DA et al. Med Phys (2024).**
* Barrigel, made from NASHA, is indicated for rectal spacing in prostate cancer patients and was FDA-cleared in May, 2022.
**Study sponsored by Palette Life Sciences, now part of Teleflex.
***Drs Gejerman, Chao, Lederer, and Orio are paid consultants of Palette Life Sciences, now part of Teleflex.
† As with any medical procedure, individual results may vary. See patient safety for more information.
Barrigel and the Barrigel logo are trademarks or registered trademarks of Teleflex Incorporated or its affiliates, in the U.S. and/or other countries.

©2025 Teleflex Incorporated. All rights reserved. APM1133A
